Lewis structure dot draw formaldehyde
Table of Contents
Table of Contents
If you’re an aspiring chemist, learning how to draw a Lewis dot structure is crucial. It’s a fundamental skill that is used to visualize the distribution of electrons in a compound or molecule. Without it, you’ll be unable to determine the chemical properties of a substance or even comprehend chemical reactions. Fortunately, with a little practice and some guidance, mastering the skill is as easy as pie.
Drawing a Lewis dot structure may seem daunting, but don’t worry, you’re not alone. Many students struggle with this task, and it’s not uncommon to make errors or even feel lost. Some students may even despise it because they find it time-consuming or challenging. One common pain point students experience is identifying the number of valence electrons present in an atom, which is necessary knowledge for drawing a Lewis dot structure. Others may find it challenging to know where the electrons go or how to depict the ions. Whatever the case, it’s essential to take a step back, breathe, and approach it methodically.
Before we dive into the step-by-step guide on drawing a Lewis dot structure, let’s brush up on a few basic concepts. First, let’s define what a Lewis dot structure is. It’s a model that shows how valence electrons are arranged among atoms in a molecule. They are depicted as dots or dashes around the atom symbol. The number of valence electrons an atom has is related to its position in the periodic table, and elements like carbon, oxygen, and nitrogen are known to form multiple bonds; hence, they can be drawn using Lewis dot structures.
To draw a Lewis dot structure, we must first determine the type of bond the atoms form (ionic or covalent). Once we know the type of bond, we can then determine the number of valence electrons for each atom, and then begin placing the electrons around the atoms using the following rules:
How to Draw a Lewis Dot Structure: A Step-by-Step Guide
My experience with drawing Lewis dot structures has been challenging but fascinating. I always thought that chemistry was hard, but with this skill, I have come to appreciate the beauty that lies in understanding how atoms bond together to form different molecules.
To begin, let’s first determine the type of bond the atoms form. If it’s an ionic bond, the electrons are transferred, whereas covalent bonds share valence electrons between the atoms.
Suppose we’re drawing the Lewis dot structure for methane (CH4). In that case, we know that carbon (C) has four valence electrons while hydrogen (H) has one. We can represent carbon as C and the four hydrogen atoms as H. Next, we place H at four points around C to represent the four covalent bonds. Finally, we can draw dots around the H atoms to depict their lone pairs of electrons.
 If you scratch your head when drawing a Lewis dot structure, don’t worry—everyone makes mistakes when learning. With some practice, you’ll be able to draw them with your eyes closed.
If you scratch your head when drawing a Lewis dot structure, don’t worry—everyone makes mistakes when learning. With some practice, you’ll be able to draw them with your eyes closed.
What happens when atoms have odd numbers of electrons?
It’s common to encounter odd numbers of valence electrons in atoms. In these instances, one electron is placed on each atom before doubling up. Take nitrogen (N), for example, which has five valence electrons. Drawing the Lewis dot structure for nitrogen involves placing a single dot on each atom before pairing them up.
 ### Beyond the Basics: What are Formal Charges?
### Beyond the Basics: What are Formal Charges?
Formal charges are a useful tool for determining the most appropriate Lewis dot structure when there are several possible ways to depict it. They help us understand which structure is best by tracking electron density and showing which atoms have a positive or negative charge. To calculate, you must subtract the number of valence electrons in the free atom from the total number of electrons occupying the atom in the Lewis structure.
Electron Deficient Compounds
Electron-deficient compounds contain fewer electrons than atoms. As a result, it can be challenging to draw their Lewis dot structures. Such compounds, such as boron trifluoride (BF3), require us to use empty orbitals instead of single electrons, which can create stability issues.
The Advantages of Knowing How to Draw a Lewis Dot Structure
Knowing how to draw a Lewis dot structure equips you with an excellent analytical skill set that allows you to better comprehend how different substances interact. This skill is helpful for determining the structure, bonding, and even properties of various molecules. It’s also essential for passing any chemistry exam, and most importantly, it’s the foundation for many advanced chemistry concepts.
Question and Answer
Q: How do you depict the ions in a Lewis dot structure?
A: Ions are depicted by adding or subtracting dots and dashes depending on whether they are positively or negatively charged.
Q: What happens when there are more than two atoms in a compound?
A: You’ll need to first determine which atoms bond together, form double or triple bonds, and then calculate the number of valence electrons. After that, it’s a matter of depicting the atoms, assigning the single electrons, and bonding them using covalent bonds.
Q: Is it possible to draw a Lewis dot structure for every molecule?
A: In some cases, it’s not possible to draw a Lewis dot structure for each molecule. For instance, some substances, such as chlorine trifluoride, contain an odd number of electrons, making the structure ambiguous.
Q: Is there an easier way to determine Formal Charges?
A: Yes, you can use this formula: Formal Charge = Valence electrons - non-bonding electrons - 1/2 number of bonding electrons.
Conclusion of How to Draw a Lewis Dot Structure
In conclusion, learning how to draw a Lewis dot structure is fundamental for any aspiring chemist. It’s a skill that helps you understand how atoms interact and form molecules. While it can be challenging at first, with some practice and patience, anyone can master the technique. Hopefully, this guide has given you a better understanding and a headstart on drawing Lewis dot structures. So go ahead, grab your pencil and paper, and start practicing!
Gallery
Lewis Structure | Chemistry Classroom, Teaching Chemistry, Chemistry

Photo Credit by: bing.com / lewis structure dot draw chemistry molecular structures infographic notes worksheet organic classroom help simple geometry drawing diagram electron worksheets babe
Chemistry Notes

Photo Credit by: bing.com /
How To Draw A Lewis Structure

Photo Credit by: bing.com / lewis structure dot draw formaldehyde
How To Draw Lewis Dot Structure
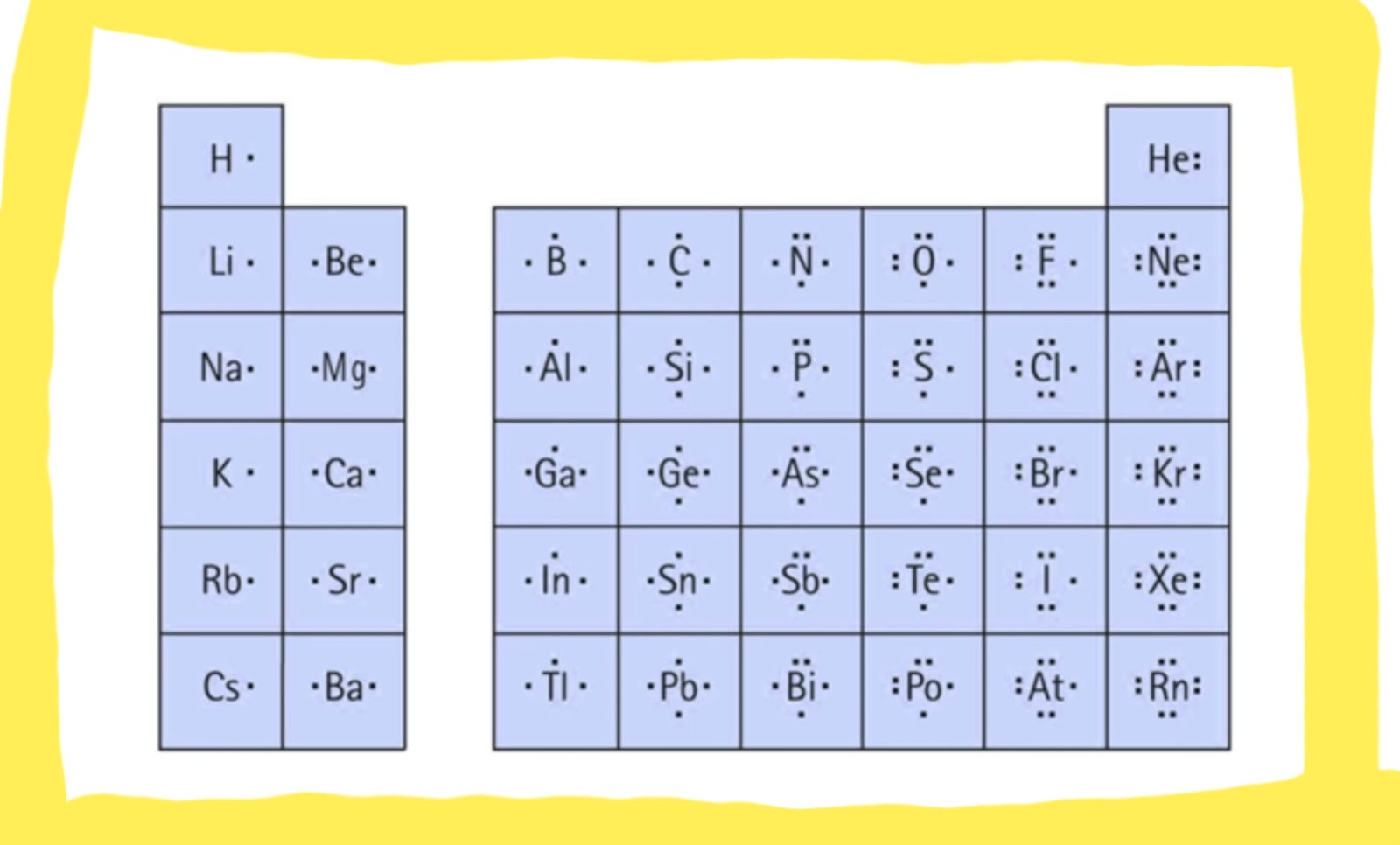
Photo Credit by: bing.com / lewis dot structure structures diagram valence draw electrons drawing represent often used number
Lewis Diagrams Made Easy: How To Draw Lewis Dot Structures - YouTube

Photo Credit by: bing.com / lewis dot draw structures diagrams easy made





